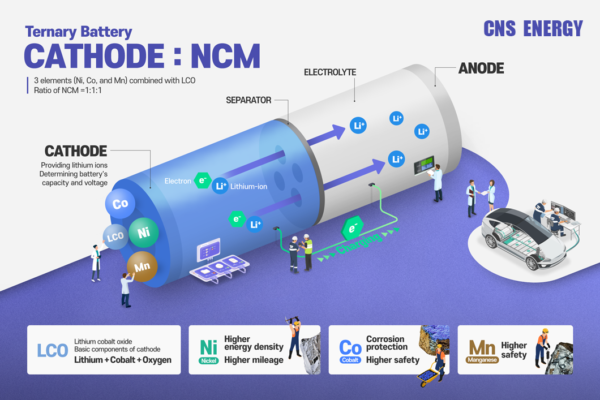
Electrolytes are crucial components in a wide range of applications, from batteries to biochemical processes. Understanding the different types of electrolytes and their unique characteristics can greatly enhance the performance and efficiency of various systems. This guide aims to provide a comprehensive overview of electrolytes, optimized for search engine visibility and user engagement.
1. Introduction to Electrolytes
Electrolytes are substances that produce an electrically conducting solution when dissolved in a polar solvent, such as water. They are essential for the operation of various devices and systems, including batteries, fuel cells, and biological functions. The primary role of electrolytes is to facilitate the movement of ions, which is critical for maintaining electrical balance and enabling chemical reactions.
2. Types of Electrolytes
Liquid Electrolytes
Liquid electrolytes are the most commonly used type and are found in a variety of applications, particularly in batteries and electrochemical cells. They typically consist of a solvent and a dissolved salt, acid, or base.
Examples:
Aqueous Electrolytes: Solutions of salts (like NaCl), acids (like HCl), or bases (like NaOH) in water.
Non-Aqueous Electrolytes: Solutions using organic solvents such as ethylene carbonate, propylene carbonate, or dimethyl carbonate, often used in lithium-ion batteries.
Characteristics:
High Ionic Conductivity: Allows efficient ion transport.
Ease of Preparation: Simple mixing of solvents and solutes.
Versatility: Can be tailored for specific applications by changing the solvent or solute.
Solid Electrolytes
Solid electrolytes are materials that conduct ions in a solid-state, providing a safer and more stable alternative to liquid electrolytes. They are increasingly used in advanced battery technologies.
Examples:
Ceramic Electrolytes: Such as lithium lanthanum zirconium oxide (LLZO) and lithium phosphorus oxynitride (LiPON).
Polymer Electrolytes: Solid polymers like polyethylene oxide (PEO) doped with lithium salts.
Characteristics:
Enhanced Safety: Reduced risk of leakage and flammability.
High Mechanical Stability: Maintains integrity under stress.
Wide Temperature Range: Effective across a broad spectrum of temperatures.
Gel Electrolytes
Gel electrolytes combine the properties of liquid and solid electrolytes, offering flexibility and enhanced safety. They are typically used in applications requiring flexible or form-fitting energy storage solutions.
Examples:
Polymer Gels: Polymers such as polyacrylonitrile (PAN) or polyvinylidene fluoride (PVDF) mixed with liquid electrolytes.
Ionic Liquid Gels: Gels formed with ionic liquids and polymer matrices.
Characteristics:
Flexibility: Can be molded into various shapes.
Good Ionic Conductivity: Comparable to liquid electrolytes.
Improved Safety: Lower risk of leakage compared to pure liquid electrolytes.
3. Characteristics of Electrolytes
Conductivity
High Ionic Conductivity: Essential for efficient ion transport in batteries and fuel cells. Liquid electrolytes generally offer higher conductivity than solid and gel counterparts.
Temperature Dependence: Conductivity can vary with temperature; solid electrolytes often perform better at higher temperatures.
Stability
Chemical Stability: Resistance to degradation and chemical reactions. Solid electrolytes typically offer superior chemical stability.
Thermal Stability: Ability to maintain performance across a wide temperature range. Solid electrolytes often provide better thermal stability.
Compatibility
Electrode Compatibility: Electrolytes must be compatible with electrode materials to avoid side reactions and ensure efficient operation.
Environmental Compatibility: Non-toxic and environmentally friendly electrolytes are preferred for sustainable applications.
4. Applications of Electrolytes
Batteries: Electrolytes are critical in lithium-ion, nickel-metal hydride, and lead-acid batteries.
Fuel Cells: Used in proton exchange membrane (PEM) fuel cells and solid oxide fuel cells (SOFCs).
Electrochemical Capacitors: Utilized in supercapacitors and ultracapacitors.
Biological Systems: Essential for nerve function and muscle contraction.
5. Comparative Analysis
Liquid vs. Solid Electrolytes
Safety: Solid electrolytes are generally safer due to reduced flammability.
Conductivity: Liquid electrolytes typically offer higher conductivity.
Stability: Solid electrolytes provide better chemical and thermal stability.
Solid vs. Gel Electrolytes
Flexibility: Gel electrolytes offer more flexibility and form-fitting capabilities.
Conductivity: Solid electrolytes may have lower ionic conductivity compared to gels.
6. Conclusion
Electrolytes play a vital role in the performance and safety of various electrochemical devices. Understanding the types and characteristics of electrolytes can help in selecting the appropriate material for specific applications, thereby enhancing efficiency and reliability.
7. References
“Electrolytes in Electrochemical Devices,” Journal of Electrochemical Science, 2022.
“Advances in Solid Electrolytes for Batteries,” Materials Today, 2021.
“Polymer and Gel Electrolytes in Energy Storage,” Energy Storage Materials, 2020.
By understanding the various types of electrolytes and their properties, industry professionals and consumers can make informed decisions that optimize the performance and safety of their devices. This comprehensive guide serves as a valuable resource for anyone looking to delve deeper into the world of electrolytes.